2006
Magnus Rueping, Cengiz Azap
Angew. Chem. Int. Ed. 2006, 45, 7832-7835
ABSTRACT:
A Mannich–aza-Michael reaction in which both an electrophile and a nucleophile are activated provides a simple, highly enantioselective route to isoquinuclidines (1) from imines (2) and cyclohexenone (3). A feature of the reaction is the interplay of an achiral and a chiral Brønsted acid, which enable the asymmetric reaction process by cooperative activation of the enone and the imine.
8. Chiral induction from solvents—lactic acid esters in the asymmetric hydroboration of ketones
Stefan H. Hüttenhain, Martin U. Schmidt, Fenja R. Schoepke, Magnus Rueping
Tetrahedron 2006, 62, 12420-12423
ABSTRACT:
The hydroboration of acetophenone in the chiral solvent (S)-methyl lactate exhibits moderate enantioselectivities. A six-membered transition state involving the ketone, the borane, and the lactate as the only chiral source is proposed. Molecular modeling explains the experimentally observed enantioselectivities. Calculated ee-values are in accordance with those experimentally observed. Improved ee-values (up to 60%) can be obtained in the presence of stoichiometric amounts of Lewis acid at lower reaction temperatures.
Magnus Rueping, Andrey P. Antonchick, Thomas Theissmann
Angew. Chem. Int. Ed. 2006, 45, 6751-6755
ABSTRACT:
A highly efficient transfer hydrogenation of benzoxazines, benzothiazines, and benzoxazinones with as low as 0.01 mol % binol phosphate catalyst furnishes the dihydro-2H-benzoxazines, -benzothiazines, and -benzoxazinones in good yields and with excellent enantioselectivities (see scheme). Particularly noteworthy are the mild reaction conditions and the wide scope of substrates, including sulfur-containing compounds.
6. Efficient Metal-Catalyzed Hydroarylation of Styrenes
Magnus Rueping, Boris J. Nachtsheim, Thomas Scheidt
Org. Lett. 2006, 8, 3717-3719
ABSTRACT:
A highly efficient metal-catalyzed hydroarylation of various styrenes has been developed. This new bismuth-catalyzed C−H functionalization provides straightforward access to a series of valuable 1,1-diarylalkane products.
Magnus Rueping, Andrey P. Antonchick, Thomas Theissmann
Angew. Chem. Int. Ed. 2006, 45, 3683-3686
ABSTRACT:
A Brønsted acid catalyzed cascade transfer hydrogenation provides direct access to 2-aryl- and 2-alkyl-substituted tetrahydroquinolines with excellent enantioselectivities under mild conditions and using very low amounts of catalyst (see scheme). The best results were achieved with the binol phosphate catalyst, where Ar=9-phenanthryl, used successfully in the Strecker reaction.
4. An Effective Bismuth‐Catalyzed Benzylation of Arenes and Heteroarenes
Magnus Rueping, Boris J. Nachtsheim, Winai Ieawsuwan
Adv. Syn. Catal. 2006, 348, 1033-1037
ABSTRACT:
A highly efficient Bi(OTf)3-catalyzed benzylation of arenes and heteroarenes has been developed. The mild reaction conditions, high yields, operational simplicity and practicability, broad scope, and remarkably low catalyst loading render this environment friendly process an attractiv approach to diarylmethane derivatives. The extension to an intramolecular variant of this procedure provides a valuable route to substituted fluorenes.
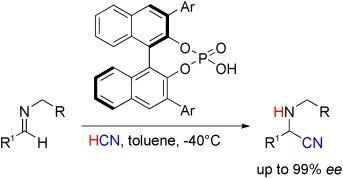
3. A Highly Enantioselective Brønsted Acid Catalyst for the Strecker Reaction
Magnus Rueping, Erli Sugiono, Cengiz Azap
Angew. Chem. Int. Ed. 2006, 45, 2617-2619
ABSTRACT:
An optimized new chiral binol phosphate catalyst (see scheme; Ar=9-phenanthryl) for the hydrocyanation of imines provides a convenient strategy for the enantioselective synthesis of α-amino acids and diamines.
Magnus Rueping, Thomas Theissmann, Andrey P. Antonchick
Synlett 2006, 1071-1074
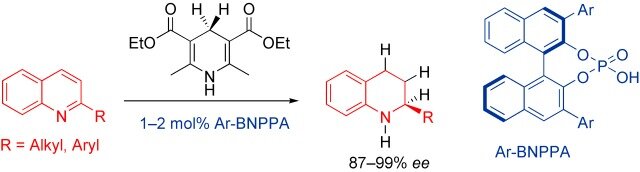
ABSTRACT:
The first metal-free Brønsted acid catalyzed hydrogenation of quinolines using Hantzsch dihydropyridine as the hydrogen source has been developed. This, so far unprecedented organocatalytic reduction of heteroaromatic compounds provides a variety of differently substituted 1,2,3,4-tetrahydroquinolines in excellent yields under mild reaction conditions using a remarkably low amount of Brønsted acid catalyst.
1. Aldol Reactions within the RNA World
Magnus Rueping
Angew. Chem. Int. Ed. 2006, 45, 1838-1840
ABSTRACT:
It is hard to find new aspects to the aldol reaction, as a wealth of diastereoselective and enantioselective examples with a range of catalysts are known. However, recent work on template-mediated cross-aldol reactions and ribozyme-catalyzed aldol reactions show that this topic is still hot. Both reports are relevant to the theory of the RNA world.