2021
16. Magnesium complexes in hydroelementation and reduction catalysis: Opportunities and challenges
Marc Magre, Marcin Szewczyk, Magnus Rueping
Curr. Opin. Green Sustain. Chem. 2021, 32, 100526
ABSTRACT:
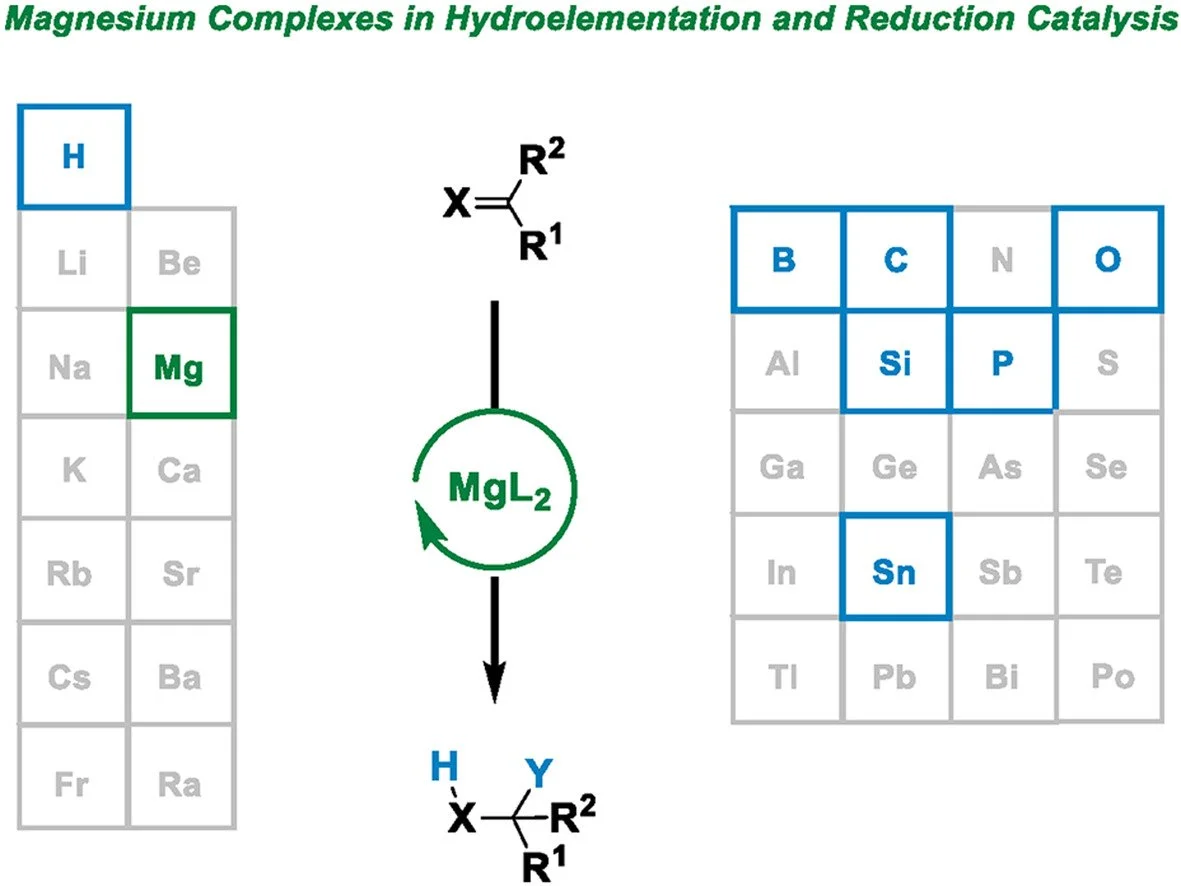
The addition of a Y–H (Y = B, Si, Sn, N, P, and O) bond and H2 to unsaturated bonds is a powerful and atom-economic method for the synthesis of fine chemicals. In the recent years, magnesium-based organometallic complexes have appeared as an alternative to transition metal catalysts for the hydrofunctionalization and hydrogenation of unsaturated systems. This review focuses on the progress of magnesium catalysis for the hydrofunctionalization and hydrogenation of unsaturated bonds, provides a critical assessment of the state-of-the-art research, highlights the major developments achieved in the past three years, and provides an overview of the challenges and opportunities.
Krishnamoorthy Muralirajan, Rajesh Kancherla, Jeremy A Bau, Mayur Rahul Taksande, Muhammad Qureshi, Kazuhiro Takanabe, Magnus Rueping
ACS Catal. 2021, 11, 24, 14772–14780
ABSTRACT:
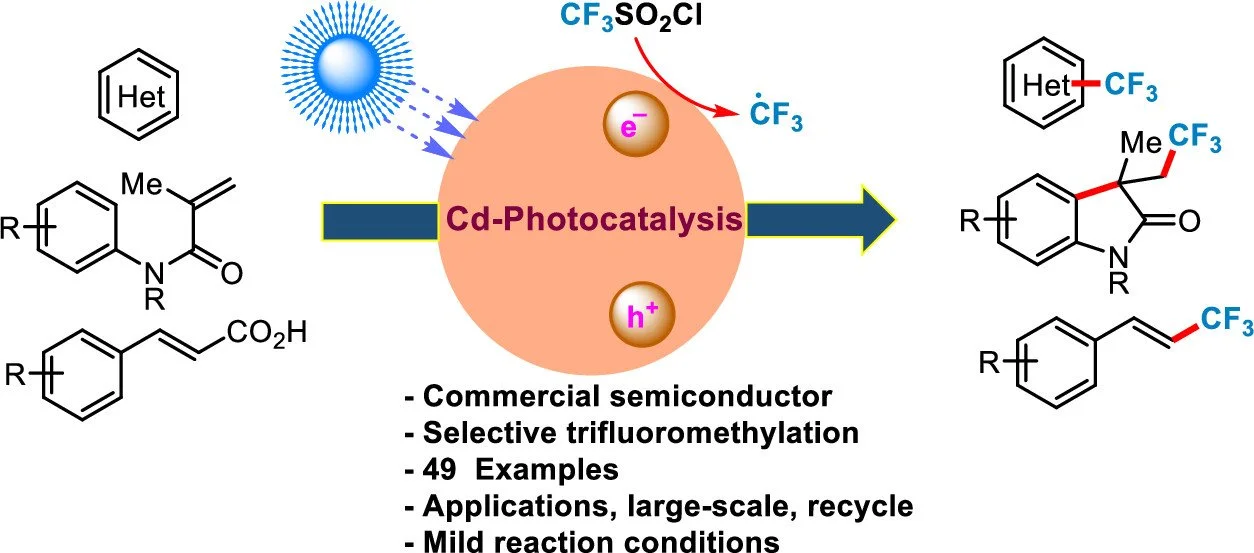
The field of heterogeneous photoredox catalysis has grown substantially and impacted organic synthesis because of the affordability and reusability of catalysts. This study reports radical trifluoromethylation with Cd–chalcogenide semiconductors. Cd semiconductors, particularly CdSe, are readily available, commercial, visible-light-responsive, heterogeneous photocatalysts. The potential of readily available Cd semiconductors, particularly CdSe, is confirmed by their increased photocatalytic activity toward trifluoromethylation with various substrates, such as (hetero)arenes and vinylic amides/acids, via addition, cyclization, and decarboxylation under visible light. The economic significance of this strategy is also highlighted through the scalable synthesis of biologically active molecules followed by catalyst reuse. Moreover, these catalysts are relatively inexpensive compared with transition metal-based homogeneous photocatalysts, presently used in organic synthesis.
14. Toward Liquid Phase Processable Metal Organic Frameworks: Dream or Reality?
Daria Poloneeva, Shuvo Jit Datta, Luis Garzon-Tovar, Sara Durini, Magnus Rueping, Mohamed Eddaoudi, Anastasiya Bavykina, Jorge Gascon
Acc. Mater. Res. 2021, 2, 1133–1140
ABSTRACT:
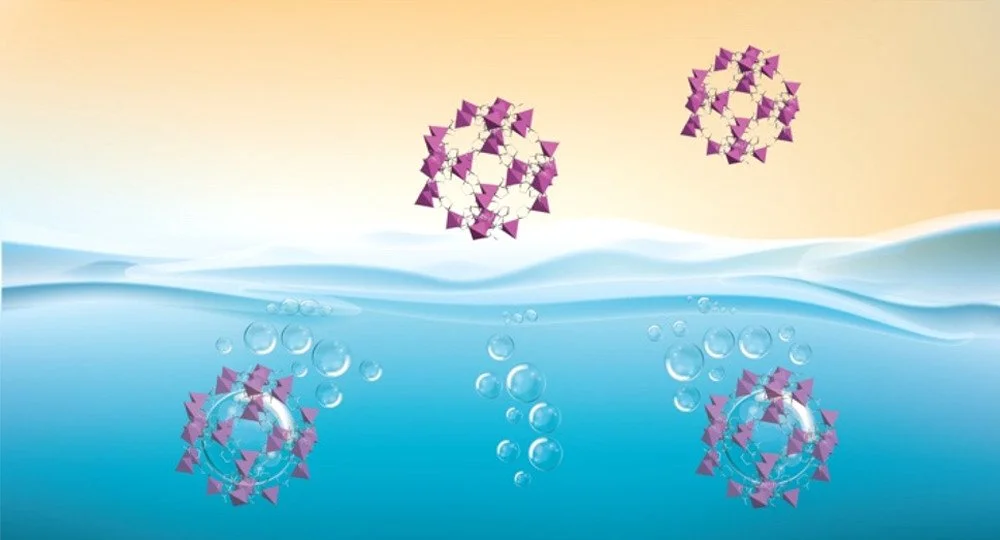
Conventionally, the virtue of porosity is only given to porous solids. Metal Organic Frameworks (MOFs), carbon materials, or zeolites are some examples. However, processing these solids is not a straightforward task. Here, we discuss how to endow porous solids (MOFs) with liquid phase processability. More specifically, we show that surface modification of MOF crystals can lead to the formation of porous liquids (PLs) that can be further processed in the liquid phase. For instance, when placed in mesitylene, ZIF-67 predictably sediments. In contrast, with the adequate surface modification, stable dispersion of ZIF-67 can be achieved. Our proposed surface modification is facile and rapid. N-Heterocyclic carbenes are chosen as modifying agents as they are similar to imidazole linkers present on ZIFs. A simple stirring of a MOF and carbene mixture results in a modified solid. The morphology and textural properties of the modified MOF do not change from the ones of its parent. Since the porosity in solution remains unoccupied, the obtained stable colloids behave as porous liquids. Research into porous liquids is an emerging field that has already shown great promise in gases storage. Our breakthrough experiments show that these particular PLs have large potential for the separation of CO2/CH4 mixtures.
The surface functionalized ZIF-67 could also be coprocessed with polymers to yield highly loaded Mixed-Matrix Membranes (MMMs) that cannot be achieved with a pristine MOF. Dispersions of functionalized ZIF-67 were blended with 6FDA-DAM and other homemade polymers in a shape of MMMs. While MMMs based on a pure MOF maintain good physical resistance at low loadings, increasing the concentration of MOF results in brittle composites. In contrast, MMMs made from functionalized ZIF retain good mechanical strength even at ca. 47.5 wt % loadings. Such high loading was possible to achieve due the better dispersion of the MOF particles during MMM fabrication and to the better affinity of the modified MOF with the polymer. The results obtained for this MMM are among the best MMMs ever reported for high challenging C3H6/C3H8 separation.
The method is not limited to ZIF-67 but can be applied to a large body of MOFs that are constructed from imidazole-based linkers, as shown in this Account. The factors that determine whether a PL is formed include, but are not limited to, surface to volume ratio, framework particle size, and topology. On top of that, we propose potential strategies for the expansion of this method by carefully choosing surface modifiers that will suit other families of MOFs.
Bholanath Maity, Chen Zhu, Magnus Rueping, Luigi Cavallo
ACS Catal. 2021, 11, 13973–13982
ABSTRACT:
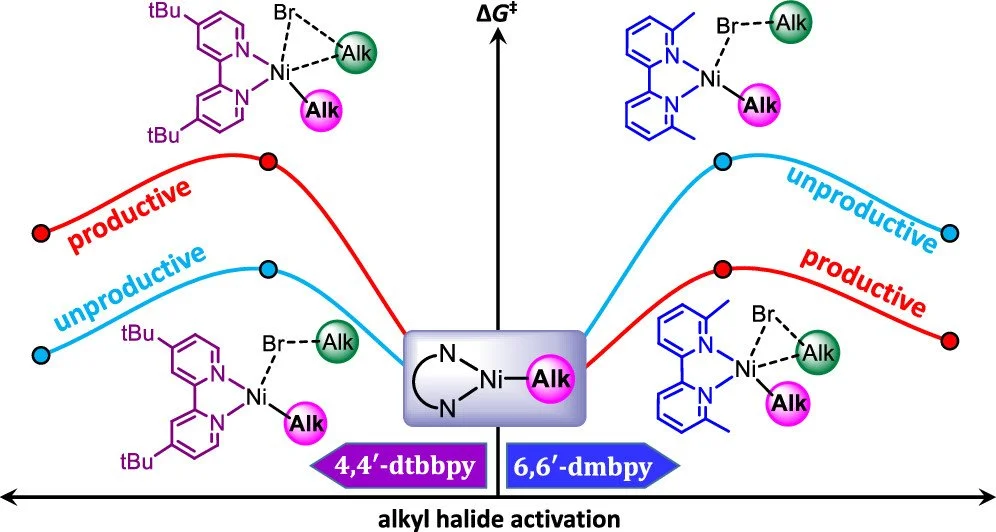
Computational analysis of the arylation of aliphatic Csp3–H bonds catalyzed by decatungstate–nickel validates the reaction mechanism and provides valuable insights into how to accomplish the corresponding alkylation. Our analysis indicates that the light-excited decatungstate photocatalyst activates one of the Csp3–H bonds by a hydrogen atom transfer reaction involving the bridging O atoms of decatungstate. The generated alkyl radical reacts with a bipyridine-based Ni catalytic species to form a NiI-alkyl intermediate. Oxidative addition of aryl bromide to this intermediate leads to a NiIII complex, from which the cross-coupling product is liberated via reductive elimination. We also investigated the corresponding prototype reaction where the aryl bromide is replaced by an alkyl bromide, which suggested a too high energy barrier for the oxidative addition of the alkyl bromide to the NiI-alkyl intermediate. Variation of the steric and electronic properties of the bipyridine ligand suggests that steric encumbrance in the 6,6′ position can promote oxidative addition of the alkyl bromide as verified by ad hoc experimental tests.
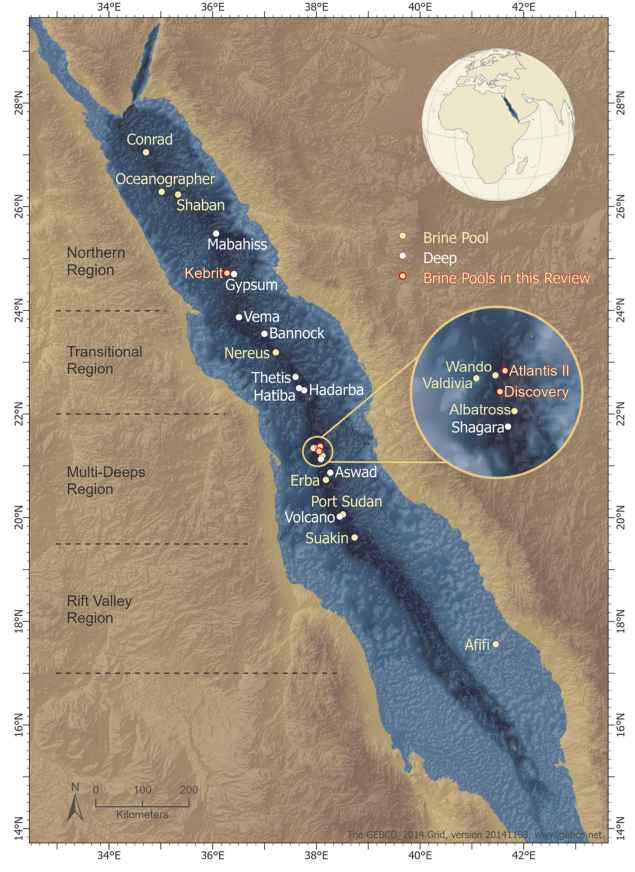
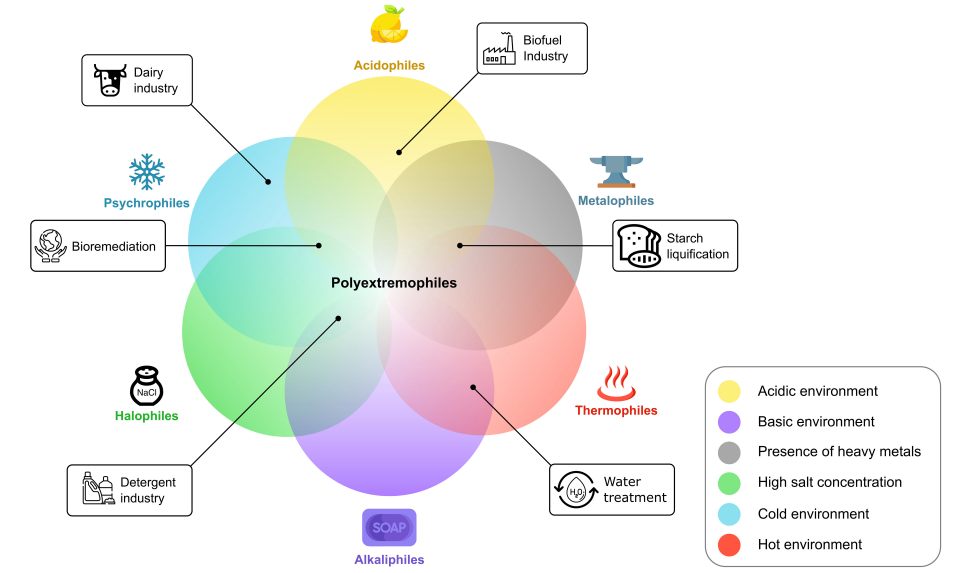
12. Novel enzymes from the red sea brine pools: current state and potential
Dominik Renn, Lera Shepard, Alexandra Vancea, Ram Karan, Stefan T Arold, Magnus Rueping
Front. Microbiol. 2021, 12, 732856
ABSTRACT:
The Red Sea is a marine environment with unique chemical characteristics and physical topographies. Among the various habitats offered by the Red Sea, the deep-sea brine pools are the most extreme in terms of salinity, temperature and metal contents. Nonetheless, the brine pools host rich polyextremophilic bacterial and archaeal communities. These microbial communities are promising sources for various classes of enzymes adapted to harsh environments – extremozymes. Extremozymes are emerging as novel biocatalysts for biotechnological applications due to their ability to perform catalytic reactions under harsh biophysical conditions, such as those used in many industrial processes. In this review, we provide an overview of the extremozymes from different Red Sea brine pools and discuss the overall biotechnological potential of the Red Sea proteome.
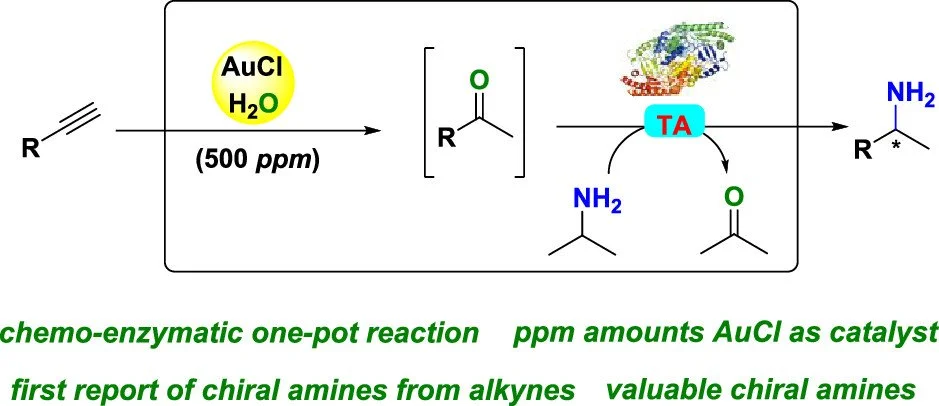
11. One-pot chemoenzymatic conversion of alkynes to chiral amines
Sam Mathew, Arunachalam Sagadevan, Dominik Renn, Magnus Rueping
ACS Catal. 2021, 11, 12565–12569
ABSTRACT:
A one-pot chemoenzymatic sequential cascade for the synthesis of chiral amines from alkynes was developed. In this integrated approach, just ppm amounts of gold catalysts enabled the conversion of alkynes to ketones (>99%) after which a transaminase was used to catalyze the production of biologically valuable chiral amines in a good yield (up to 99%) and enantiomeric excess (>99%). A preparative scale synthesis of (S)-methylbenzylamine and (S)-4-methoxy-methylbenzylamine from its alkyne form gave a yield of 59 and 92%, respectively, with ee > 99%
10. Unactivated alkyl chloride reactivity in excited-state palladium catalysis
Krishnamoorthy Muralirajan, Rajesh Kancherla, Aidana Gimnkhan, Magnus Rueping
Org. Lett. 2021, 23, 6905–6910
ABSTRACT:
Excited-state palladium catalysis is an efficient process for the alkylation of diverse organic compounds via the generation of alkyl radicals from alkyl bromides and iodides. However, the generation of alkyl radicals from more stable alkyl chlorides remains challenging. Herein, we demonstrate the excited-state palladium-catalyzed synthesis of oxindoles and isoquinolinediones via alkylation/annulation reaction by overcoming inherent limitations associated with unactivated C(sp3)–Cl bond activation at room temperature.
Joost Steverlynck, Ruzal Sitdikov, Magnus Rueping
Chem. Eur. J. 2021, 27, 11751-11772
ABSTRACT:
In the field of medicinal chemistry, the precise installation of a trideuteromethyl group is gaining ever-increasing attention. Site-selective incorporation of the deuterated “magic methyl” group can provide profound pharmacological benefits and can be considered an important tool for drug optimization and development. This review provides a structured overview, according to trideuteromethylation reagent, of currently established methods for site-selective trideuteromethylation of carbon atoms. In addition to CD3, the selective introduction of CD2H and CDH2 groups is also considered. For all methods, the corresponding mechanism and scope are discussed whenever reported. As such, this review can be a starting point for synthetic chemists to further advance trideuteromethylation methodologies. At the same time, this review aims to be a guide for medicinal chemists, offering them the available C−CD3 formation strategies for the preparation of new or modified drugs.
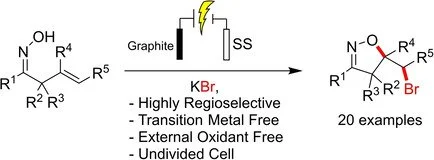
Ajit Prabhakar Kale, Pavlo Nikolaienko, Kristina Smirnova, Magnus Rueping
Eur. J. Org. Chem. 2021, 2021, 3496-3500
ABSTRACT:
A highly regioselective protocol for the synthesis of isoxazolines through cascade C−O and C−Br bond formation has been developed. The electrochemical approach uses traceless electrons as a sole source of oxidant, thus avoiding the use of stoichiometric organic or inorganic oxidants and provides a mild and environmentally benign alternative pathway for the synthesis of a wide range of valuable substituted isoxazolines from alkenyl oximes in good yields.
Jeremy A Bau, Magnus Rueping
Chem. Eur. J. 2021, 27, 8960-8965
ABSTRACT:
Hydrogen storage in the form of intermediate artificial fuels such as methanol is important for future chemical and energy applications, and the electrochemical regeneration of hydrogen from methanol is thermodynamically favorable compared to direct water splitting. However, CO produced from methanol oxidation can adsorb to H2-evolution catalysts and drastically reduce activity. In this study, we explore the origins of CO immunity in Mo-containing H2-evolution catalysts. Unlike conventional catalysts such as Pt or Ni, Mo-based catalysts display remarkable immunity to CO poisoning. The origin of this behavior in NiMo appears to arise from the apparent inability of CO to bind Mo under electrocatalytic conditions, with mechanistic consequences for the H2-evolution reaction (HER) in these systems. This specific property of Mo-based HER catalysts makes them ideal in environments where poisons might be present.
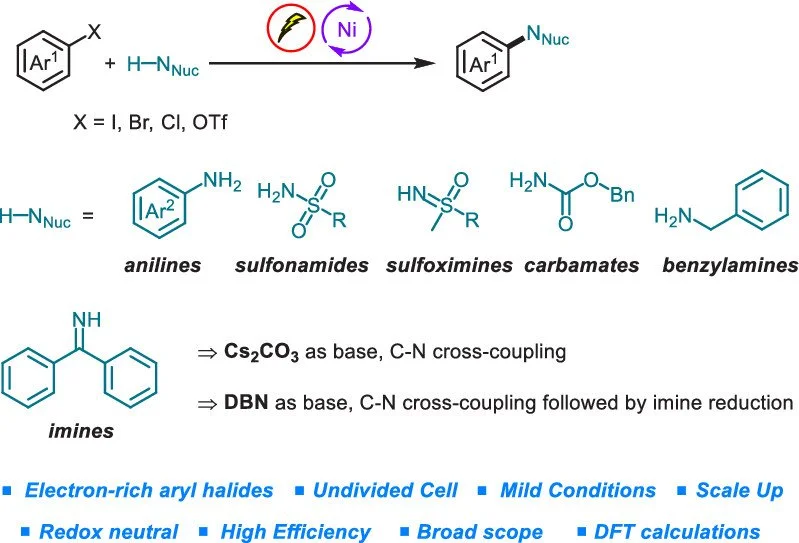
Chen Zhu, Ajit Prabhakar Kale, Huifeng Yue, Magnus Rueping
JACS Au 2021, 1, 1057–1065
ABSTRACT:
A nickel-catalyzed cross-coupling amination with weak nitrogen nucleophiles is described. Aryl halides as well as aryl tosylates can be efficiently coupled with a series of weak N-nucleophiles, including anilines, sulfonamides, sulfoximines, carbamates, and imines via concerted paired electrolysis. Notably, electron-deficient anilines and sulfonamides are also suitable substrates. Interestingly, when benzophenone imine is applied in the arylation, the product selectivity toward the formation of amine and imine product can be addressed by a base switch. In addition, the alternating current mode can be successfully applied. DFT calculations support a facilitated reductive elimination pathway.
5. Air stable iridium catalysts for direct reductive amination of ketones
Iuliia Polishchuk, Jan Sklyaruk, Yury Lebedev, Magnus Rueping
Chem. Eur. J. 2021, 27, 5919-5922
ABSTRACT:
Half-sandwich iridium complexes bearing bidentate urea-phosphorus ligands were found to catalyze the direct reductive amination of aromatic and aliphatic ketones under mild conditions at 0.5 mol % loading with high selectivity towards primary amines. One of the complexes was found to be active in both the Leuckart–Wallach (NH4CO2H) type reaction as well as in the hydrogenative (H2/NH4AcO) reductive amination. The protocol with ammonium formate does not require an inert atmosphere, dry solvents, as well as additives and in contrast to previous reports takes place in hexafluoroisopropanol (HFIP) instead of methanol. Applying NH4CO2D or D2 resulted in a high degree of deuterium incorporation into the primary amine α-position
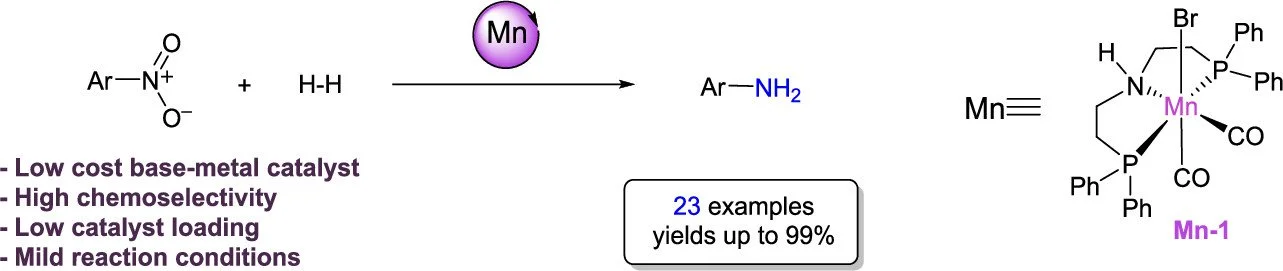
4. Chemoselective hydrogenation of nitroarenes using an air-stable base-metal catalyst
Viktoriia Zubar, Abhishek Dewanji, Magnus Rueping
Org. Lett. 2021, 23, 2742–2747
ABSTRACT:
The reduction of nitroarenes to anilines as well as azobenzenes to hydrazobenzenes using a single base-metal catalyst is reported. The hydrogenation reactions are performed with an air-and moisture-stable manganese catalyst and proceed under relatively mild reaction conditions. The transformation tolerates a broad range of functional groups, affording aniline derivatives and hydrazobenzenes in high yields. Mechanistic studies suggest that the reaction proceeds via a bifunctional activation involving metal–ligand cooperative catalysis.
Chen Zhu, Huifeng Yue, Jiaqi Jia, Magnus Rueping
Angew. Chem. Int. Ed. 2021, 60, 17810-17831
ABSTRACT:
The formation of C-heteroatom bonds represents an important type of bond-forming reaction in organic synthesis and often provides a fast and efficient access to privileged structures found in pharmaceuticals, agrochemical and materials. In contrast to conventional Pd- or Cu-catalyzed C-heteroatom cross-couplings under high-temperature conditions, recent advances in homo- and heterogeneous Ni-catalyzed C-heteroatom formations under mild conditions are particularly attractive from the standpoint of sustainability and practicability. The generation of NiIII and excited NiII intermediates facilitate the reductive elimination step to achieve mild cross-couplings. This review provides an overview of the state-of-the-art approaches for mild C-heteroatom bond formations and highlights the developments in photoredox and nickel dual catalysis involving SET and energy transfer processes; photoexcited nickel catalysis; electro and nickel dual catalysis; heterogeneous photoredox and nickel dual catalysis involving graphitic carbon nitride (mpg-CN), metal organic frameworks (MOFs) or semiconductor quantum dots (QDs); as well as more conventional zinc and nickel dual catalyzed reactions.
2. Bioprospecting of novel extremozymes from prokaryotes—the advent of culture-independent methods
Maksim Sysoev, Stefan W Grötzinger, Dominik Renn, Jörg Eppinger, Magnus Rueping, Ram Karan
Front. Microbiol. 2021, 12, 630013
ABSTRACT:
Extremophiles are remarkable organisms that thrive in the harshest environments on Earth, such as hydrothermal vents, hypersaline lakes and pools, alkaline soda lakes, deserts, cold oceans, and volcanic areas. These organisms have developed several strategies to overcome environmental stress and nutrient limitations. Thus, they are among the best model organisms to study adaptive mechanisms that lead to stress tolerance. Genetic and structural information derived from extremophiles and extremozymes can be used for bioengineering other nontolerant enzymes. Furthermore, extremophiles can be a valuable resource for novel biotechnological and biomedical products due to their biosynthetic properties. However, understanding life under extreme conditions is challenging due to the difficulties of in vitro cultivation and observation since > 99% of organisms cannot be cultivated. Consequently, only a minor percentage of the potential extremophiles on Earth have been discovered and characterized. Herein, we present a review of culture-independent methods, sequence-based metagenomics (SBM), and single amplified genomes (SAGs) for studying enzymes from extremophiles, with a focus on prokaryotic (archaea and bacteria) microorganisms. Additionally, we provide a comprehensive list of extremozymes discovered via metagenomics and SAGs.
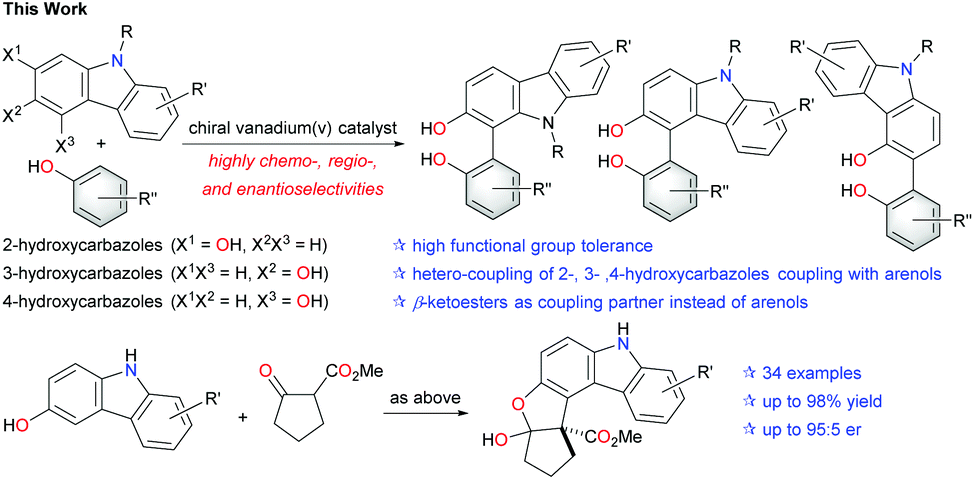
Makoto Sako, Keigo Higashida, Ganesh Tatya Kamble, Kevin Kaut, Ankit Kumar, Yuka Hirose, Da-Yang Zhou, Takeyuki Suzuki, Magnus Rueping, Tomohiro Maegawa, Shinobu Takizawa, Hiroaki Sasai
Org. Chem. Front., 2021, 8, 4878-4885
ABSTRACT:
The catalytic enantioselective oxidative hetero-coupling of arenols using a chiral vanadium(V) complex has been developed. The coupling of hydroxycarbazole derivatives with various arenols provided axially chiral biarenols with high chemo-, regio-, and enantioselectivities. The reaction took place under mild conditions and exhibited satisfactory functional group tolerance. Aerobic oxidative hetero-coupling with β-ketoesters also proceeded with high chemo- and stereoselectivities under slightly modified reaction conditions.