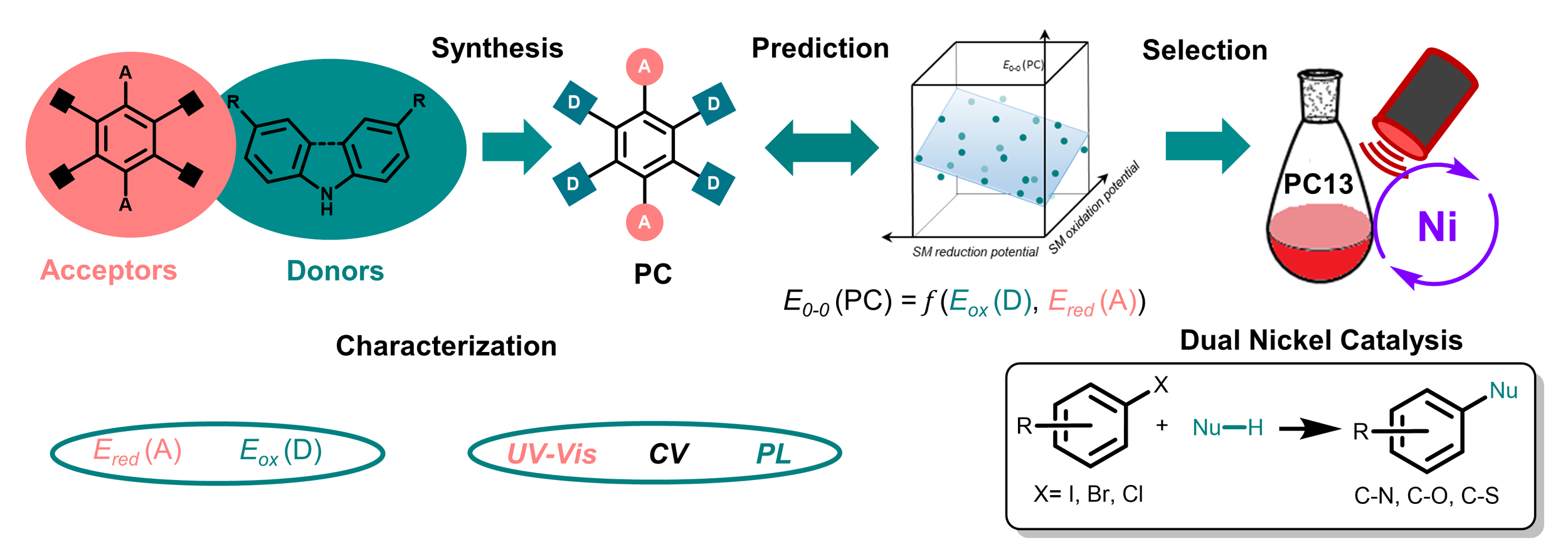
Predictive, Data-Driven Design of Red-Light Photoredox Catalysts for C─Heteroatom Bond Formation
Amir Gizatullin, Dr. Tingting Yuan, Dr. Sascha Grotjahn, Prof. Dr. Luigi Cavallo, Prof. Dr. Burkhard König, Prof. Dr. Chen Zhu, Prof. Dr. Magnus Rueping
Angew. Chem. Int. Ed. 2026, e26086
DOI: 10.1002/anie.202526086
Abstract:
Photocatalysis is a powerful tool for the synthesis of organic molecules, yet its widespread application is hindered by the dependence on high-energy light sources and expensive metal-based catalysts, which can limit scalability and environmental sustainability. In this study, we present a modular design strategy for organic dyes engineered for efficient red-light absorption, enabling photocatalytic reactions under low-energy irradiation. Our findings establish a clear relationship between the oxidation potential of the photocatalyst and the nature of its donor moiety, as well as between the reduction potential and the electronic characteristics of its core structure. Moreover, we demonstrate that the E0-0 energy of a photocatalyst can be predicted via multivariate linear regression using the donor's oxidation potential and the core's reduction potential as descriptors. Utilizing this strategy, we synthesized red-light-absorbing photocatalysts that efficiently promote C─heteroatom cross-coupling reactions under mild conditions. This approach overcomes the limitations of blue-light photocatalysis by offering broad substrate compatibility, including π-conjugated aryl bromides and photolabile functional groups, while minimizing undesirable hydrodehalogenation. By reducing reliance on precious metals and improving energy efficiency, our approach provides a scalable alternative to traditional photocatalysis and advances the development of metal-free photocatalysts for sustainable chemistry.
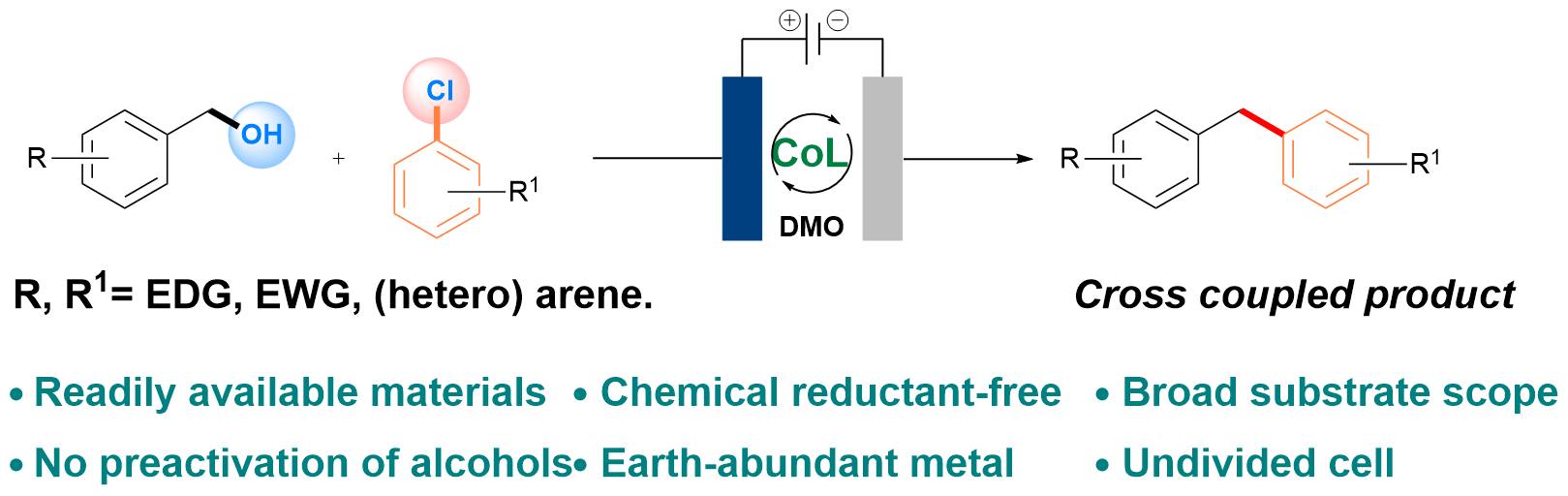
Prashant S. Shinde, Valmik S. Shinde, Magnus Rueping
Chem. Sci., 2026, 17, 652-663
DOI: 10.1039/D5SC05919D
Abstract:
The direct functionalization of alcohols via C–O bond cleavage is a synthetically valuable but challenging transformation. In this work, we report a reductive C(sp3)–C(sp2) cross-coupling reaction between benzyl alcohols and a broad range of aryl chlorides. The success of this transformation is attributed to the development of low-coordinate cobalt/bipyridine complexes, which enable the selective conversion of benzyl alcohols into the corresponding diarylmethanes, while minimizing undesired homocoupling of either the benzyl alcohol or aryl chlorides.
Moyu Yi, Karthik Peramaiah, Xingzhu Chen, Renqian Zhou, Nursaya Zhumabay, Hongbin Dou, Hao Huang , Indranil Dutta, Shouwei Zuo, Jifeng Wu, Bin Chang, Tsu-Chien Weng, Magnus Rueping, Osman M. Bakr, Huabin Zhang, Kuo-Wei Huang
Appl. Catal. B: Environ., 2026, 383, 126126
DOI: 10.1016/j.apcatb.2025.126126
Abstract:
Activating catalytically inert planar sites and achieving high-rate CO2 to formic acid conversion remain key challenges for industrial implementation of bismuth nanoflower-based electrocatalyst. Here, we integrate in-situ reconstruction of Bi nanoclusters with heterointerfacial electric fields at a Bi/Cu junction. The dual (geometric + electronic) activation creates secondary cluster sites, enhances interfacial charge transformation, and improves the selectivity to the key product formic acid. The resulting catalyst achieves > 87 % Faradaic efficiency across a wide current density range (100–600 mA cm−2) and enables mole-scale production of pure formic acid (≈ 0.16 mol) over 100 h of continuous operation in a solid electrolyte reactor. In-situ studies and DFT calculations reveal a synergistic mechanism of planar surface activation by cluster engineering and electric field-enhanced intermediate binding. This work establishes a scalable and integrative catalyst-interface-reactor framework for practical CO2 electrolysis toward a liquid hydrogen carrier.
Resonant acoustic mixing enables solvent-less amide coupling in solid-phase peptide synthesis
Alice Nanni, Panayiotis Bilalis, Magnus Rueping
Green Chem., 2026, 28, 255-263
DOI: 10.1039/D5GC04067A
Abstract:
Solid-phase peptide synthesis (SPPS) is the backbone of modern peptide production. However, it relies heavily on relatively toxic solvents and generates significant waste, limiting its sustainability and scalability. To address these limitations, we report the first fully solvent-less peptide coupling protocol for SPPS enabled by Resonant Acoustic Mixing (RAM), representing a step toward greener peptide manufacturing. This method eliminates bulk solvent use, reagent pre-dissolution, and pre-activation during coupling by using mechanical agitation to drive efficient amide bond formation. Optimized conditions (95g acceleration, 5 min, 1.5 equiv. Fmoc-amino acids) afford rapid and clear reactions with high conversion and purity. Notably, no external solvent is added during coupling; instead, residual solvent retained from resin pre-swelling creates a localized microenvironment sufficient for in situ activation. Compared to conventional SPPS, this protocol significantly reduces solvent and reagent use, reaction time, and waste. Process Mass Intensity (PMI) calculations show clear improvements, highlighting the method's environmental and economic benefits. This approach was validated by synthesizing two bioactive peptides (IKVAV and Angiotensin 1–7) in high yield and purity, and further demonstrated excellent scalability in a tenfold scale-up.