Rajesh Kancherla, Krishnamoorthy Muralirajan, Sayan Dutta, Kuntal Pal, Bo Li, Bholanath Maity, Luigi Cavallo, Magnus Rueping
Angew. Chem. Int. Ed. 2023, e202314508
DOI: 10.1002/anie.202314508
Abstract:
The development of metal complexes that function as both photocatalyst and cross-coupling catalyst remains a challenging research topic. So far, progress has been shown in palladium(0) excited-state transition metal catalysis for the construction of carbon-carbon bonds where the oxidative addition of alkyl/aryl halides to zero-valent palladium (Pd0) is achievable at room temperature. Distinct from Pd0-complexes that easily undergo oxidative addition with aryl/alkyl halides, the oxidative addition of aryl halides to electrophilic divalent Pd(II) is uphill and energy-demanding. Overcoming these limitations, we realized the oxidative addition of aryl halides to divalent palladium using mild photochemical conditions at room temperature under open air conditions. Various electron withdrawing and donating aryl/heteroaryl iodides were cross-coupled with sodium azide to give primary anilines by using Pd(OAc)2 as both photocatalyst and cross-coupling catalyst. We demonstrate for the first time, that divalent PdII can act as a light-absorbing species which undergoes double excitation to give the C−N cross-coupled product under air. DFT studies and mechanistic investigations support the photoexcitation of two distinct divalent palladium species and unravel the reaction mechanism.
Shao-Chi Lee, Chen Zhu, Kun Huang, Jeremy A Bau, Jiaqi Jia, Huifeng Yue, Magnus Rueping
ACS Catal. 2023, 13, 16279–16285
DOI: 10.1002/anie.202314508
Abstract:
We describe a novel and efficient photoinduced nickel-catalyzed strategy for the demethylative cyanation as well as decarboxylative cyanomethylation of aryl halides is presented. Notably, the use of NiBr2·dtbbpy complex as a catalyst and commercially available bromoacetonitrile as a cyanating reagent eliminates the need for a photocatalyst, making the cya-nation reaction a practical protocol for nitril synthesis. Furthermore, employing cesium cyanoacetate as the cy-anomethylation reagent, the combination of photoredox and nickel dual catalysis enables the successful decarboxy-lative cyanomethylation of aryl halides under mild reaction conditions. This method offers a versatile approach for the synthesis of a wide range of aryl and benzyl nitriles, incor-porating diverse functional groups, with good to excellent efficiency. The results of the control experiments including EPR experiments, Stern-Volmer quenching, and radical-trap studies, support the photoinduced catalysis mechanisms.
Liang Yi, Chen Zhu, Xiangyu Chen, Huifeng Yue, Tengfei Ji, Yiqiao Ma, Yuanyuan Cao, Rajesh Kancherla, Magnus Rueping
Chem. Sci., 2023,14, 14271-14279
DOI: 10.1039/D3SC04410F
Abstract:
We have developed the first visible-light-mediated photoredox and HAT dual catalyzed oxyalkylation of unactivated alkenes of β,γ-unsaturated oximes. This strategy produces a wide range of isoxazoline derivatives through reactions with different Michael acceptors, styrenes, or α-CF3 alkenes. Our approach successfully addresses the high redox potential of the oxime, complements the activation of the O–H bond by quinuclidine, and provides a versatile pathway to generate iminoxyl radicals. Furthermore, this efficient and mild synthetic methodology has the potential to produce a diverse array of valuable isoxazoline compounds, which may contribute to advancements in drug discovery efforts. Further studies may be directed towards the further exploration of photoredox HAT dual catalysis generated iminoxyl radicals in synthesis and reaction development.
Jean Michél Merkes, Tarushyam Mukherjee, Silvia Chiera, Olli-Ville Laukkanen, Joerg Bruenke, Tim Brust, Francesco Tessarolo, Fabian Kiessling, Magnus Rueping, Srinivas Banala
Adv. Mater. Interfaces 2023, 2300601.
Abstract:
During the COVID-19 pandemic, the use of polypropylene fleece-based personal protection equipment (PPE) increased significantly to over ten million tons. Typically, most PPEs are discarded after a single use, to prevent self-infection of users and spread of infectious agents. However, in order to minimize plastic waste without compromising the protective properties of PPE, it is crucial to explore new reusable or longer-lived materials. Here, a visible light-activatable antimicrobial photodynamic dye coating for PPEs is presented. In this context, coating with thiomorpholino-methylene blue (TMB), derived from methylene blue by introducing two thiomorpholine units, is found to show high antibacterial activity. TMB was integrated into the rotary printing suspension, the concentration of TMB was optimized, and it was found that 5% TMB is suitable for coatings to reduce the number of Gram-positive and -negative bacteria by 99.99% after 6 h of white light irradiation. Bacterial filtration efficiency and breathability tested according to EN 14683, confirmed that the TMB coating does not affect the filter performance. Thus, this antimicrobial photodynamic dye coating technique offers a promising solution for a safer and extended use of PPE, and reduction of plastic waste generated by PPEs.
Zeyad M. Abdulhamid, Aasif A. Dabbawala, Thomas Delclos, Rainer Straubinger, Magnus Rueping, Kyriaki Polychronopoulou, Dalaver H. Anjum
Sci Rep 2023, 13, 19705
DOI: 10.1038/s41598-023-46960-w
Abstract:
This work presents a hydrothermal-based facile method for synthesizing ZnFe2O4, whose size can be controlled with the concentration of sodium acetate used as a fuel and its physical changes at nanoscales when exposed to two different gases. The structural, morphological, compositional, and electronic properties of the synthesized samples are also presented in this paper. The crystal structure of the synthesized samples was determined using an X-ray Diffractometer (XRD). The results revealed fluctuations in the size, lattice parameter, and strain in the nanoparticles with increasing the concentration of sodium acetate. Field-Emission Scanning Electron Microscopy (FESEM) was used to determine synthesized materials’ morphology and particle size. It revealed that the particles possessed approximately spherical morphology whose size decreased significantly with the increasing amount of sodium acetate. Transmission Electron Microscopy (TEM) was utilized to determine the structure, morphology, and elemental distributions in particles at the nanoscale, and it confirmed the findings of XRD and FESEM analyses. The high-resolution TEM (HRTEM) imaging analysis of the nanoparticles in our studied samples revealed that the particles predominantly possessed (001) type facets. X-ray photoelectron spectroscopy (XPS) and core-loss electron energy loss spectroscopy (EELS) showed an increasing fraction of Fe2+ with the decreasing size of the particles in samples. The Brunauer, Emmett, and Tellers (BET) analysis of samples revealed a higher surface area as the particle size decreases. In addition, the determined surface area and pore size values are compared with the literature, and it was found that the synthesized materials are promising for gas-sensing applications.
Bo Li, Liang Yi, Bholanath Maity, Jiaqi Jia, Yuqin Shen, Xiang-Yu Chen, Luigi Cavallo, Magnus Rueping
ACS Catal. 2023, 13, 15194–15202
DOI: 10.1021/acscatal.3c04601
Abstract:
Noncovalent interactions play fundamental roles in many organic and biochemical processes, including hydrogen and halogen bonding, which are vital in the fields of synthesis and catalysis. Herein, we describe the application of halogen bonding interactions to facilitate the reaction balance between an electron donor–acceptor (EDA) complex and Ph2Se2 for the synthesis of a series of organoselenium compounds. The EDA complex concept has recently emerged as an attractive path for visible light-induced transformations due to facile reaction conditions and the avoidance of photocatalysts. Density functional theory calculations reveal that an iodine–π* interaction leads to the formation of an alkyl radical from the N-(acyloxy)phthalimide ester. The resulting alkyl radical is captured by the diselenide–I• complex to form the C–Se bond. The developed selenide synthesis strategy has been applied in the transformation of primary, secondary, and tertiary carboxylic acids as well as a series of natural products, drugs, and α-selenoamino acids.
Krishnamoorthy Muralirajan, Rajesh Kancherla, Bholanath Maity, Safakath Karuthedath, Frédéric Laquai, Luigi Cavallo, Magnus Rueping
Nat Commun. 2023, 14, 6622, DOI: 10.1038/s41467-023-42392-2
Abstract:
Photocatalytic selective C(sp3)–H activation/cross-coupling reactions are appealing in organic synthesis. In this manuscript, we describe the development of photoexcited-state Pd-catalyzed dehydrogenative β-sulfonylation reactions using amines and aryl sulfonyl chlorides via intermolecular hydrogen atom transfer (HAT) and C−S cross-coupling processes at room temperature. The transformation can be achieved by the direct generation of two distinct Pd-radical hybrid species and their capability to promote two different reactivities from Pd(0) and aryl sulfonyl chlorides, allowing for the efficient conversion of readily available amines into stable sulfonyl-substituted enamines at room temperature. The in-depth experimental, computational, and transient optical spectroscopic study and catalytic applications of a dehydrogenative functionalization event provide evidence for both static and dynamic quenching, as well as inner-sphere and outer-sphere mechanisms. This methodology can be applied to the selective dehydrogenation of more complex molecules and new cross-coupling transformations. The mechanistic insight of the reactive species is also of relevance to the further development of photoexcited-state Pd-catalysis and will inspire the design of tailored catalysts and excited-state catalysis reactions.
Kathiravan Murugesan, Arunachalam Sagadevan, Lu Peng, Oleksandr Savateev, Magnus Rueping
ACS Catal. 2023, 13, 13414–13422, DOI: 10.1021/acscatal.3c03798
Abstract:
The conversion of feedstock materials into useful chemical compounds through feasible processes is highly sought after from both industrial and environmental perspectives. In this study, we developed a simple and scalable protocol for synthesizing aldehydes, ketones, and amides from abundant raw materials, such as alkanes, alkenes, and carboxylic acids. Using a photoactive mesoporous graphitic carbon nitride catalyst (mpg-CN) and molecular oxygen, we successfully transformed over 60 substrates in good yield and selectivity. This method is suitable for the late-stage modification of existing drug molecules into their corresponding carbonyl derivatives and can provide direct access to intermediates or oxidative decomposition and degradation products important for understanding biological pathways in drug development. Additionally, our protocol is applicable for large-scale preparation, and the mpg-CN catalyst can be recycled without significant loss of catalytic activity and selectivity. Comprehensive experiments, including labeled oxygen studies, support the photo-oxidative mechanism.
Electrochemical cobalt catalysis enabled construction of diverse chiral skeletons via C–H activation
Huifeng Yue, Chen Zhu, Magnus Rueping
Sci. Bull. 2023, 68, DOI: 10.1016/j.scib.2023.07.028
Abstract:
The development of general, efficient methods for constructing chiral compounds using cost-effective catalysts and eco-friendly catalytic platforms remains a high priority. Recently, an elegant enantioselective synthetic strategy employing electrochemical cobalt catalysis for the aryl C-H activation of benzamides and phosphinic amides has been reported. This approach facilitates the synthesis of a diverse range of C-stereogenic, atropoisomeric, and P-stereogenic skeletons with high chemo-, regio-, and enantioselectivities. This work offers valuable insights for electro-oxidative transformations and is expected to find widespread applications in asymmetric synthesis.
Chunwei Dong, Ren-Wu Huang, Arunachalam Sagadevan, Peng Yuan, Luis Gutiérrez-Arzaluz, Atanu Ghosh, Saidkhodzha Nematulloev, Badriah Alamer, Omar F. Mohammed, Irshad Hussain, Magnus Rueping, Osman M. Bakr
Angew. Chem. Int. Ed. 2023, 62, e202307140
Abstract:
Elucidating single-atom effects on the fundamental properties of nanoparticles is challenging because single-atom modifications are typically accompanied by appreciable changes to the overall particle’s structure. Herein, we report the synthesis of a [Cu58H20PET36(PPh3)4]2+(Cu58; PET: phenylethanethiolate; PPh3: triphenylphosphine) nanocluster—an atomically precise nanoparticle—that can be transformed into the surface-defective analog [Cu57H20PET36(PPh3)4]+(Cu57). Both nanoclusters are virtually identical, with five concentric metal shells, save for one missing surface copper atom in Cu57. Remarkably, the loss of this single surface atom drastically alters the reactivity of the nanocluster. In contrast to Cu58, Cu57shows promising activity for click chemistry, particularly photoinduced [3+2] azide–alkyne cycloaddition (AAC), which is attributed to theactive catalytic site in Cu57after the removal of one surface copper atom. Our study not only presents a unique system for uncovering the effect of a single-surface atom modification on nanoparticle properties but also showcases single-atom surface modification as a powerful means for designing nanoparticle catalysts.
Chen Zhu, Haifeng Chen, Huifeng Yue, Magnus Rueping
Nature Synthesis, 2023, 2, 1068–1081, DOI: 10.1038/s44160-023-00349-9
Abstract:
The development of general and efficient strategies for the construction of allenes is important due to their wide applications. Although few protocols have been developed via the 1,4-difunctionalization of 1,3-enynes under thermal or photoredox conditions, the mild and robust methodology for dicarbofunctionalization and hydroalkylation remains unexplored. In the present study, we report an electrochemical multicomponent protocol for the chemo- and regioselective difunctionalization of 1,3-enynes. In particular, 1,4-arylalkylation and unsymmetrical dialkylation have been realized via electro- and nickel dual catalysis using graphite/nickel foam and zinc/nickel foam as electrodes, respectively. The use of a Zn/reticulated vitreous carbon electrode led to efficient 1,4-hydro(deutero)alkylation in the absence of a metal catalyst. A wide range of structurally diverse tri- and tetra-substituted allenes were easily prepared with good efficiency and excellent regioselectivity under mild reaction conditions. Notably, a series of natural product- and drug-derived substrates could undergo late-stage functionalization to generate the corresponding complex allenes.
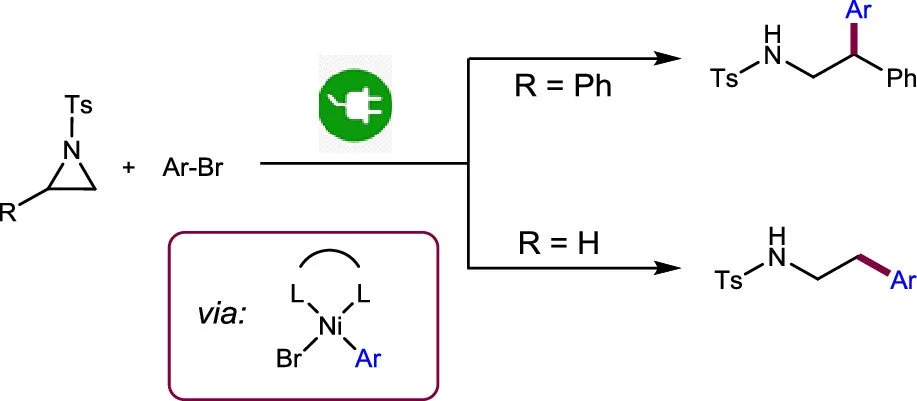
Gadde Sathish Kumar, Chen Zhu, Rajesh Kancherla, Prashant S. Shinde, Magnus Rueping
ACS Catal. 2023, 13, 8813–8820
Abstract:
An efficient method for the reductive cross-electrophile coupling of aziridines with aryl halides using nickel electrocatalysis is reported. This reaction provides valuable phenethylamines at room temperature in good yields. The reaction was successfully applied to the electro-reductive cross-coupling of less reactive, unsubstituted N-tosylaziridine. Additionally, we were even able to couple challenging alkyl bromides with aryl aziridines. Control experiments and voltammetric studies confirm that the mechanism is distinct from conventional and photochemical couplings. Furthermore, the role of both Ni(II) organometallic complexes and electrodes was examined, providing insight into the reaction mechanism and the beneficial role of the sacrificial anode in electrochemical cross-couplings.
Haifeng Chen, Chen Zhu, Huifeng Yue, Magnus Rueping
Angew. Chem. Int. Ed. 2023, 62, e202306498
Abstract:
The difunctionalization of unsaturated bonds plays a vital role in the enrichment of molecular complexity. While various catalytic methods for alkene and alkyne difunctionalization have been developed in recent years, hetero-functionalization the introduction of two different atoms has been less explored. This is mainly due to the challenges associated with achieving high chemo-, regio-, and stereoselectivity, especially when adding two similar atoms from the same group across unsaturated bonds. In this study, we describe a nickel-catalyzed, three-component reductive protocol for group 14 element hetero-difunctionalization of 1,3-enynes using electrochemistry. This new method is mild, selective, and general, allowing for the silyl-, germanyl-, and stannyl-alkylation of enynes. Various chlorosilanes as well as chlorogermans, and chlorostannanes can be successfully used in combination with aryl/alkyl-substituted 1, 3-enynes and primary, secondary, and tertiary alkyl bromides in the electroreductive coupling.
Metallaphotoredox catalysis for sp3 C–H functionalizations through hydrogen atom transfer (HAT)
Jingchang Zhang, Magnus Rueping
Chem. Soc. Rev., 2023, 52, 4099-4120
Abstract:
In recent years, the integration of photocatalytic hydrogen atom transfer (HAT) with transition metal catalysis has emerged as a formidable strategy for the construction of C(sp3)–carbon and C(sp3)-hetero bonds. The fusion of these two methodologies has been utilized widely in organic synthesis, leading to new transformations in chemical synthesis. In this review, we aim to summarize the recent advances made in sp3 C–H functionalizations through photocatalytic HAT followed by transition metal catalysis. Our focus will be on the diverse strategies and their synthetic applications, in addition to detailed mechanisms involved in these reactions. An in-depth understanding of these mechanisms is crucial for the rational design of new catalysts and reaction conditions to further enhance the efficiency of these transformations. We hope that this review will serve as a valuable resource for researchers in the area of metallaphotoredox catalysis, and will inspire the further development of this application in green chemistry, drug synthesis, material science, and other related fields.
Carbon–Germanium Bond Formation via Low-Valent Cobalt-Catalyzed Cross-Electrophile Coupling
Haifeng Chen, Chen Zhu, Huifeng Yue, Magnus Rueping
ACS Catal. 2023, 13, 6773–6780
Abstract:
An efficient, electrochemically induced cobalt-catalyzed carbon–germanium bond formation provides access to a variety of functionalized germane-containing compounds, including aryl, vinyl, and alkyl germanes. The cobalt-catalyzed germylation is conducted under mild reaction conditions and exhibits a broad scope and functional group tolerance. Mechanistic experimental studies provide insight into the unexpected reaction pathway and the sequential activation of the different electrophiles.
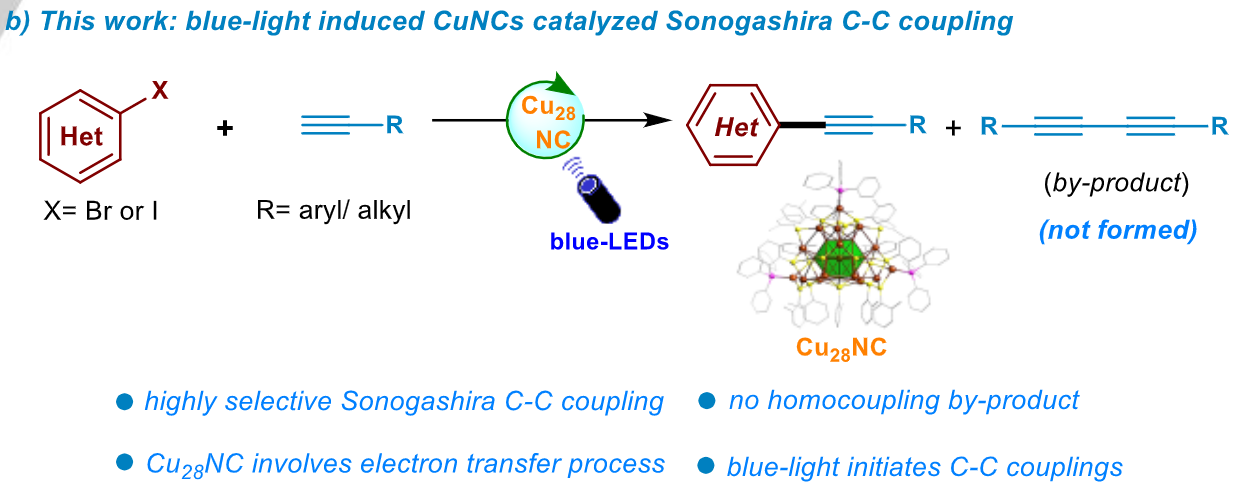
Saidkhodzha Nematulloev, Arunachalam Sagadevan, Badriah Alamer, Aleksander Shkurenko, Renwu Huang, Jun Yin, Chunwei Dong, Peng Yuan, Khursand E Yorov, Azimet A Karluk, Wasim J Mir, Bashir Hasanov, Mohamed Nejib Hedhili, Naveen Halappa, Mohamed Eddaoudi, Omar F Mohammed, Magnus Rueping, Osman M. Bakr
Angew. Chem. Int. Ed. 2023, 62, e202303572
Abstract:
Point defects in nanoparticles have long been hypothesized to play an important role in governing the particle's electronic structure and physicochemical properties. However, single point defects in material systems usually exist with other heterogeneities, obscuring the chemical role of the effects. Herein, we report the synthesis of novel atomically precise, copper hydride nanoclusters (NCs), [Cu28H10(C7H7S)18(TPP)3] (Cu28; TPP: triphenylphosphine; C7H7S: o-thiocresol) with a defined defect in the gram scale via a one-pot reduction method. The Cu28 acts as a highly selective catalyst for C−C cross-couplings. The work highlights the potential of defective NCs as model systems for investigating individual defects, correlating defects with physiochemical properties, and rationally designing new nanoparticle catalysts.
Isolated Electron Trap‐Induced Charge Accumulation for Efficient Photocatalytic Hydrogen Production
Wenhuan Huang, Chenyang Su, Chen Zhu, Tingting Bo, Shouwei Zuo, Wei Zhou, Yuanfu Ren, Yanan Zhang, Jing Zhang, Magnus Rueping, Huabin Zhang
Angew. Chem. Int. Ed. 2023, 62, e202304634
Abstract:
The solar-driven evolution of hydrogen from water using particulate photocatalysts is considered one of the most economical and promising protocols for achieving a stable supply of renewable energy. However, the efficiency of photocatalytic water splitting is far from satisfactory due to the sluggish electron-hole pair separation kinetics. Herein, isolated Mo atoms in a high oxidation state have been incorporated into the lattice of Cd0.5Zn0.5S (CZS@Mo) nanorods, which exhibit photocatalytic hydrogen evolution rate of 11.32 mmol g−1 h−1 (226.4 μmol h−1; catalyst dosage 20 mg). Experimental and theoretical simulation results imply that the highly oxidized Mo species lead to mobile-charge imbalances in CZS and induce the directional photogenerated electrons transfer, resulting in effectively inhibited electron-hole recombination and greatly enhanced photocatalytic efficiency.
Sam Mathew, Dominik Renn, Magnus Rueping
ACS Catal., 2023, 13, 5584-5598
Abstract:
Amine transaminases constitute an important class of enzymes for the synthesis of chiral amines, which are commonly used as building blocks in pharmaceutical compounds and fine chemicals. Over the past decade, developments in enzyme discovery and process and protein engineering have advanced the use of transaminases in organic synthesis. Recent advances in enzymatic cascade engineering have attracted attention due to efficient and environmentally friendly routes to synthesize chiral amines. Enzymatic cascades can reduce the traditional multistep synthesis and have enabled the generation of chiral products from cheap starting materials. This review focuses on chiral amine synthesis by enzymatic and chemoenzymatic cascades utilizing amine transaminases.
Nickel-Сatalyzed Carbon–Selenium Bond Formations under Mild Conditions
Special Issue Honoring Masahiro Murakami’s Contributions to Science
Serik Zhumagazy, Chen Zhu, Huifeng Yue, Magnus Rueping
Synlett, 2023, 34, 1381–1384
Abstract:
A nickel-catalyzed C–Se cross-coupling between aryl iodides and selenols is described. The newly developed catalytic methodology offers facile access to various unsymmetrical selenium-containing motifs. The reaction features excellent functional group tolerance, wide substrate scope, good efficiency, and operates under mild reaction conditions. Notably, this protocol could be readily scaled up to gram scale without the loss of yield.
Bioengineering of air-filled protein nanoparticles by genetic and chemical functionalization
Ram Karan, Dominik Renn, Shuho Nozue, Lingyun Zhao, Satoshi Habuchi, Thorsten Allers, Magnus Rueping
J. Nanobiotechnology, 2023, 21, 108
Abstract:
Various bacteria and archaea, including halophilic archaeon Halobacterium sp. NRC-1 produce gas vesicle nanoparticles (GVNPs), a unique class of stable, air-filled intracellular proteinaceous nanostructures. GVNPs are an attractive tool for biotechnological applications due to their readily production, purification, and unique physical properties. GVNPs are spindle- or cylinder-shaped, typically with a length of 100 nm to 1.5 μm and a width of 30–250 nm. Multiple monomeric subunits of GvpA and GvpC proteins form the GVNP shell, and several additional proteins are required as minor structural or assembly proteins. The haloarchaeal genetic system has been successfully used to produce and bioengineer GVNPs by fusing several foreign proteins with GvpC and has shown various applications, such as biocatalysis, diagnostics, bioimaging, drug delivery, and vaccine development.
Liuzhuang Xing, Qian Yang, Chen Zhu, Yilian Bai, Yurong Tang, Magnus Rueping, Yunfei Cai
Nat. Commun. 2023, 14, 1501
Featured by the Editor: Editors’ Highlights in Catalysis
Highlighted by Synfact: Synfacts 2023, 19, 0594
Abstract:
The development of heterogeneous metallaphotocatalysis, the use of supports such as mesoporous graphitic carbon nitride (mpg-CN), is of great interest for sustainable organic synthesis. The rational design and controllable preparation of well-defined (site-isolated) metal/photo bifunctional solid catalysts to meet such goal remains a critical challenge. Herein, we demonstrate the incorporation of privileged homogeneous bipyridyl-based Ni-catalysts into highly ordered and crystalline potassium poly(heptazine imide) (K-PHI). A variety of PHI-supported cationic bipyridyl-based Ni-catalysts (LnNi-PHI) have been prepared and fully characterized by various techniques including NMR, ICP-OES, XPS, HAADF-STEM and XAS. The LnNi-PHI catalysts exhibit exceptional chemical stability and recyclability in diverse C−P, C−S, C−O and C−N cross-coupling reactions. The proximity and cooperativity effects in LnNi-PHI significantly enhances the photo/Ni dual catalytic activity, thus resulting in low catalyst loadings and high turnover numbers.
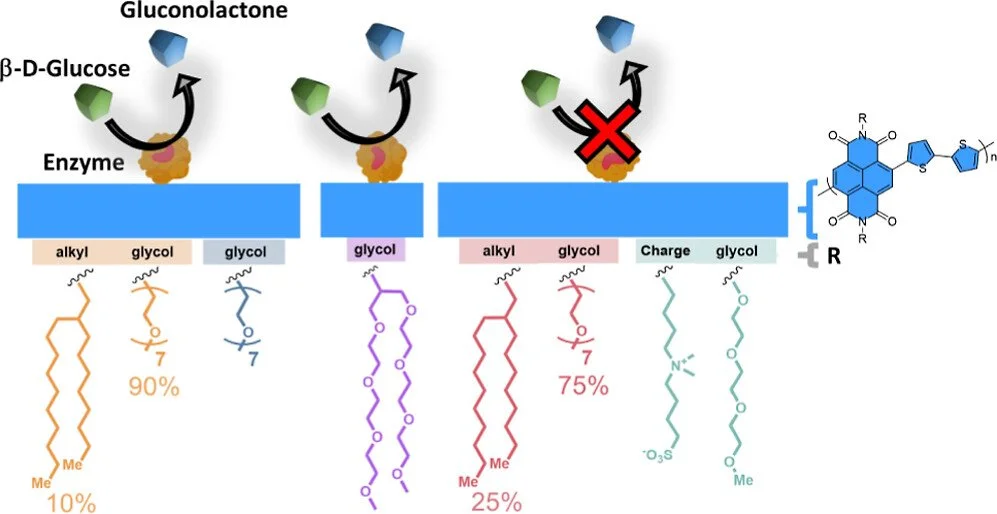
Interactions of Catalytic Enzymes with n-Type Polymers for High-Performance Metabolite Sensors
David Ohayon, Dominik Renn, Shofarul Wustoni, Keying Guo, Victor Druet, Adel Hama, Xingxing Chen, Iuliana Petruta Maria, Saumya Singh, Sophie Griggs, Bob C Schroeder, Magnus Rueping, Iain McCulloch, Sahika Inal
ACS Appl. Mater. Interfaces, 2023, 15, 9726-9739
Abstract:
The tight regulation of the glucose concentration in the body is crucial for balanced physiological function. We developed an electrochemical transistor comprising an n-type conjugated polymer film in contact with a catalytic enzyme for sensitive and selective glucose detection in bodily fluids. Despite the promise of these sensors, the property of the polymer that led to such high performance has remained unknown, with charge transport being the only characteristic under focus. Here, we studied the impact of the polymer chemical structure on film surface properties and enzyme adsorption behavior using a combination of physiochemical characterization methods and correlated our findings with the resulting sensor performance. We developed five n-type polymers bearing the same backbone with side chains differing in polarity and charge. We found that the nature of the side chains modulated the film surface properties, dictating the extent of interactions between the enzyme and the polymer film. Quartz crystal microbalance with dissipation monitoring studies showed that hydrophobic surfaces retained more enzymes in a densely packed arrangement, while hydrophilic surfaces captured fewer enzymes in a flattened conformation. X-ray photoelectron spectroscopy analysis of the surfaces revealed strong interactions of the enzyme with the glycolated side chains of the polymers, which improved for linear side chains compared to those for branched ones. We probed the alterations in the enzyme structure upon adsorption using circular dichroism, which suggested protein denaturation on hydrophobic surfaces. Our study concludes that a negatively charged, smooth, and hydrophilic film surface provides the best environment for enzyme adsorption with desired mass and conformation, maximizing the sensor performance. This knowledge will guide synthetic work aiming to establish close interactions between proteins and electronic materials, which is crucial for developing high-performance enzymatic metabolite biosensors and biocatalytic charge-conversion devices.
Huifeng Yue, Chen Zhu, Magnus Rueping
Sci. Bull. 2023, 68, 367-369
Abstract:
The transformation of simple carbohydrates into high-valueadded chemical products has always been desirable. Selective C-H bond functionalization, particularly installing valuable functionalities to the contiguous aliphatic C-H bonds simultaneously, is a highly challenging topic in organic synthesis. The work reported by Lambert, Ye, and co-workers in Nature provides an impressive example of multiple vicinal C-H oxygenations of alkyl aromatics and trifluoroacetamides via EPC, and is a significant advancement in this promising field. We believe that these findings will certainly find more applications in the preparation of complex molecules and inspire chemists to develop further efficient and mild C-H bond activation methodologies via electrophotocatalysis.
Modulating stereoselectivity in allylic C(sp3)-H bond arylations via nickel and photoredox catalysis
Long Huang, Marcin Szewczyk, Rajesh Kancherla, Bholanath Maity, Chen Zhu, Luigi Cavallo, Magnus Rueping
Nat Commun 2023, 14, 548
Abstract:
While significant progress has been made in developing selective C-H bond cross-couplings in the field of radical chemistry, the site and stereoselectivity remain a long-standing challenge. We have developed a general method that permits the regio- and stereoselective synthesis of alkenes. This protocol enables catalytic allylic radical generation via a hydrogen atom transfer (HAT) process directly from simple and readily available alkenes and accommodates a wide variety of aryl halide coupling partners. Different from previous mechanistic proposals on C(sp3)-H functionalization with photoredox nickel dual catalysis, our experimental evidence, and detailed computational mechanistic studies support a Ni(I)-allylNi(II)-allylNi(I)-Ni(III) catalytic cycle. Computational studies further explain the stereochemical outcome and indicate that excitation of a Ni(II)-allyl complex from singlet to a triplet state results in a spontaneous change of the allyl group coordination and that the subsequent ligand-dependent isomerization can be directed by choice of the ligand to achieve E/Z selectivity. These unexpected findings led us systematically investigate the stereoselectivity and to develop stereodivergent allylic C(sp3)-H bond arylations in which a ligand switch allows the target synthesis of E and Z isomers of silyl enol ethers.
An Unexpected Boron Rearrangement Leads to a Fluorogenic and Colorimetric BODIPY Probe
Jean Michel Merkes, Gerhard Raabe, Fabian Kiessling, Magnus Rueping, Srinivas Banala
Adv. Optical Mater. 2023, 2202601
Abstract:
The isolation and characterization of a kinetically stable oxadiazaborinine (ODB) dye are described, which undergoes an unexpected thermal boron rearrangement with a color change and increase in fluorescence. This controllable reaction allows for the use as a practical time-temperature indicator probe with the advantages of photostability, non-reversibility, and environmental friendliness.
Tianyu Long, Chen Zhu, Ling Li, Liang Shao, Shengqing Zhu, Magnus Rueping, Lingling Chu
Nat Commun 2023, 14, 55
Highlighted by Synfact: Synfacts 2023, 19, 0256
Abstract:
Precise stereocontrol of functionalized alkenes represents a long-standing research topic in organic synthesis. Nevertheless, the development of a catalytic, easily tunable synthetic approach for the stereodivergent synthesis of both E-selective and even more challenging Z-selective highly substituted 1,3-dienes from common substrates remains underexploited. Here, we report a photoredox and nickel dual catalytic strategy for the stereodivergent sulfonylalkenylation of terminal alkynes with vinyl triflates and sodium sulfinates under mild conditions. With a judicious choice of simple nickel catalyst and ligand, this method enables efficient and divergent access to both Z- and E-sulfonyl-1,3-dienes from the same set of simple starting materials. This method features broad substrate scope, good functional compatibility, and excellent chemo-, regio-, and stereoselectivity. Experimental and DFT mechanistic studies offer insights into the observed divergent stereoselectivity controlled by ligands.
Jose J Delgado-Marín, Alejandra Rendón-Patiño, Vijay Kumar Velisoju, Gadde Sathish Kumar, Naydu Zambrano, Magnus Rueping, Jorge Gascón, Pedro Castaño, Javier Narciso, Enrique V Ramos-Fernandez
Chem. Mater. 2023, 35, 692–699
Abstract:
Zeolitic imidazolate frameworks (ZIFs) have been profusely used as catalysts for inserting CO2 into organic epoxides (i.e., epichlorohydrin) through cycloaddition. Here, we demonstrate that these materials suffer from irreversible degradation by leaching. To prove this, we performed the reactions and analyzed the final reaction mixtures by elemental analysis and the resulting materials by different microscopies. We found that the difference in catalytic activity between three ZIF-67 and one ZIF-L catalysts was related to the rate at which the materials degraded. Particularly, the {100} facet leaches faster than the others, regardless of the material used. The catalytic activity strongly depended on the amount of leached elements in the liquid phase since these species are extremely active. Our work points to the instability of these materials under relevant reaction conditions and the necessity of additional treatments to improve their stability.
Sharath Kandambeth, Vinayak S Kale, Dong Fan, Jeremy A Bau, Prashant M Bhatt, Sheng Zhou, Aleksander Shkurenko, Magnus Rueping, Guillaume Maurin, Osama Shekhah, Mohamed Eddaoudi
Adv. Energy Mater. 2023, 13, 2202964
Abstract:
Here, this work reports an innovative strategy for the synthesis of chemically robust metal–organic frameworks (MOFs), and applies them as catalysts for the electrocatalytic oxygen evolution reaction (OER). A bimetallic squarate-based MOF (Sq-MOF) with a zbr topology serves as an excellent platform for electrocatalytic OER owing to its open porous structure, high affinity toward water, and presence of catalytically active 1D metal hydroxide strips. By regulating the Ni2+ content in a bimetallic squarate MOF system, the electrochemical structural stability toward OER can be improved. The screening of various metal ratios demonstrates that Ni3Fe1 and Ni2Fe1 Sq-zbr-MOFs show the best performance for electrocatalytic OER in terms of catalytic activity and structural stability. Ni2Fe1 Sq-zbr-MOF shows a low overpotential of 230 mV (at 10 mA cm−2) and a small Tafel slope of 37.7 mV dec−1, with an excellent long-term electrochemical stability for the OER. Remarkably, these overpotential values of Ni2Fe1 Sq-zbr-MOF are comparable with those of the best-performing layered double hydroxide (LDH) systems and outperforms the commercially available noble-metal-based RuO2 catalyst for OER under identical operational conditions.
Zheng-Jia Shen, Chen Zhu, Xiao Zhang, Chao Yang, Magnus Rueping, Lin Guo, Wujiong Xia
Angew. Chem. Int. Ed. 2023, 62, e202217244
Abstract:
(Deuterium-labeled) CF2H- and CFH2-moieties are of high interest in drug discovery. The high demand for the incorporation of these fluoroalkyl moieties into molecular structures has witnessed significant synthetic progress, particularly in the (deutero)hydrodefluorination of CF3-containing compounds. However, the controllable replacement of fluorine atoms while maintaining high chemoselectivity remains challenging. Herein, we describe the development of a selective (deutero)hydrodefluorination reaction via electrolysis. The reaction exhibits a remarkable chemoselectivity control, which is enabled by the addition of different organoboron sources. The procedure is operationally simple and scalable, and provides access in one step to high-value building blocks for application in medicinal chemistry. Furthermore, density functional theory (DFT) calculations have been carried out to investigate the reaction mechanism and to rationalize the chemoselectivity observed.